For a full-grown organism to develop from a cell, the cell should divide. Throughout the mobile division, a cell duplicates its contents and divides it into two. The primary purpose of the cell division is to precisely duplicate the organism’s DNA and its contents evenly. The cell cycle is accomplished in 4 phases with vigorous regulation at every steps. The cell division is regulated at totally different phases by varied mechanisms at cell cycle checkpoints.
In eukaryotes, the cell cycle consists of 4 phases:
- G1: cells actively develop and put together for DNA replication.
- S section: DNA replicates.
- G2: cells nonetheless develop and synthesize proteins required for division.
- M (mitosis) phase: duplicated chromosomes (sister chromatids) and cell contents divide into two daughter cells, every with a full copy of DNA.
The cell division course of can’t be random and must be extremely managed as uncontrolled division may give rise to diseased states resembling most cancers. The monitoring and upkeep of genomic integrity throughout cell cycle happens by a fancy community of DNA restore pathways and cell-cycle checkpoints. The cell cycle checkpoints play a task within the system as they detect DNA damages and, within the repose, induce cell cycle arrest till the harm will get repaired. The mechanism of motion of the cell cycle checkpoints is thru the regulation of actions of cyclins and CDKs.
Cyclins and CDKs
Cyclin is the regulatory subunit as a result of its focus adjustments as cells progress by the cell cycle. The catalytic subunit known as CDKs and switch phosphate teams from ATP to a selected amino acid residue throughout the substrates, phosphorylating them. CDKs present kinase exercise solely when related to cyclin. Phosphorylation of proteins concerned in cell-cycle management by cyclin-CDK complexes at their regulatory websites, both prompts or inhibits them.


There are various checkpoints that regulates the cell cycle; nevertheless, the three main ones are
1. G1/S (restriction) checkpoint
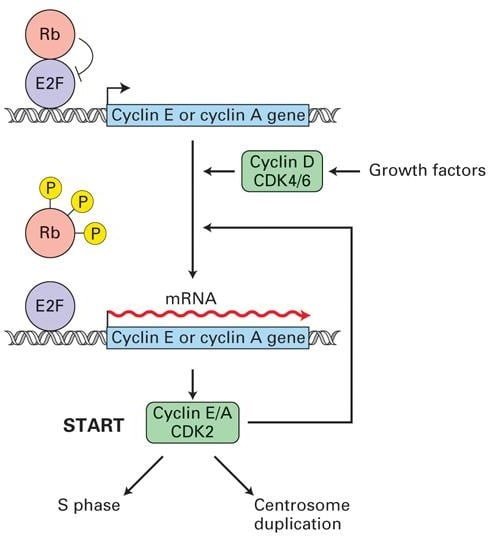
Injury to DNA, availability of vitamins, and the expansion elements are evaluated on the G1 checkpoint. If situations are insufficient, the cell will now not be allowed to enter into the S-phase. Stimulation of G0 cells with progress elements and mitogens induces expression of cyclin D, CDK4/6, and E2F transcription issue.
Function of p53 in DNA harm regulation
If there’s important DNA harm, p53 will get phosphorylated by kinases (ATM/ATR and Chk2/Chk1 kinases play a pivotal function), stopping it from binding with Mdm2. Mdm2 is a ubiquitin ligase that inhibits p53 by concentrating on it for degradation1. The p53 then stimulates the manufacturing of p21, a protein that binds and inhibits cyclin-CDK complexes, resulting in cell cycle arrest till the DNA harm will get repaired and p21 degree drops 2. If the DNA harm is irreversible, p53 triggers apoptosis (programmed cell dying), stopping the duplication of broken chromosomes.
Function of Rb protein
Rb (tumor suppressor) protein binds to and inhibits E2F expression3. When the expression of the G1 cyclin-CDKs activates, produced cyclinD-CDK4/6 complexes phosphorylate Rb protein, inactivating it and releasing the E2F transcription issue. E2F then stimulates expression of transcription genes required for entry into S-phase, particularly genes encoding DNA synthesis enzymes, and S- section cyclin-CDKs.
S-phase cyclinE/A-CDK2 advanced initially stays inhibited till the G1/S section cyclin-CDKs phosphorylate the inhibitors. After releasing, cyclinE/A-CDK2 phosphorylates regulatory proteins certain to chromosomal replication origins, selling the initiation of DNA synthesis.

2. G2 checkpoint
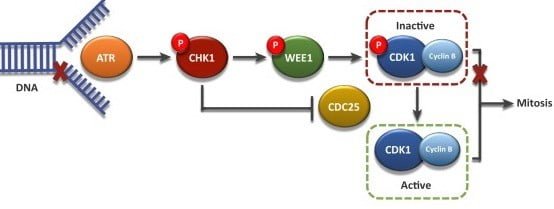
The G2 checkpoint ensures the chromosomes are replicated and the replicated DNA of the cell is just not broken earlier than getting into mitosis. Mitotic cyclin B accumulates regularly and binds to CDK1. When cyclin B binds to CDK1, the ensuing advanced is called MPF4. This advanced acts because the sign for the G2 cell to enter mitosis and induce:
- Condensation of chromosomes,
- Nuclear envelope breakdown,
- Meeting of the mitotic spindle, and
- Alignment of condensed chromosomes on the equatorial area.
Earlier than entry into mitosis, CDK1 is maintained in an inactive (phosphorylated) state by the kinase Wee1. Throughout the G2/M transition, Wee1 will get phosphorylated by PLK1 5. Phosphorylated Wee1 is then degraded by the SCF ubiquitin ligase pathway. PLK1 moreover prompts Cdc25 through phosphorylation. Tyrosine phosphatase cdc25 is chargeable for dephosphorylation and activation of CDK1 6. The compound impact of Wee1 degradation and Cdc25 activation leads to the online elimination of an inhibitory phosphate group, activating cyclinB-CDK1 advanced.

3. M checkpoint (spindle checkpoint)
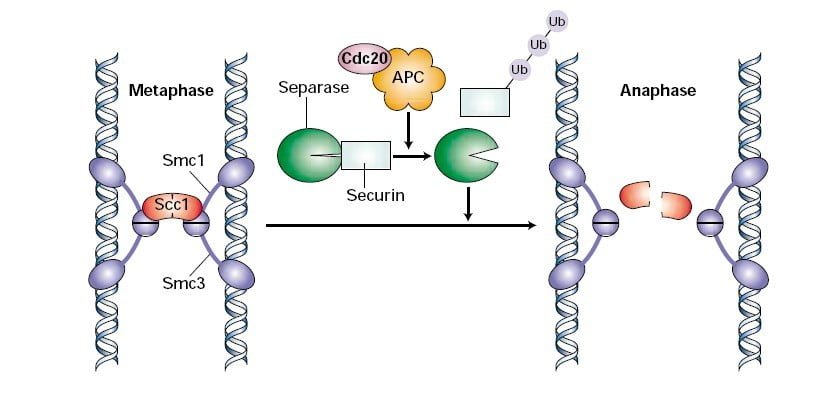
The M checkpoint is also referred to as the spindle checkpoint, as its major function is to ensure all of the sister chromatids are appropriately hooked up to the spindle microtubules. Sister chromatids fashioned by DNA replication within the S-phase are held collectively at centromeres through ring-like proteins known as cohesin advanced. Cdc20 prompts the APC/C ubiquitin ligase which is chargeable for the polyubiquitination of securin. Securin is an inhibitor of the enzyme known as separase. As soon as proteasomes degrade securin, separase cleaves the Scc1 element of the cohesin advanced, separating the sister chromatids7.
If there’s an uneven rigidity and the microtubules usually are not correctly hooked up to the chromosomes, this generates an error sign contained in the cell. Mad2 detects the error sign and inhibits the exercise of Cdc20. It delays the degradation of securin and metaphase to anaphase transition of the cell. The correct attachment of the spindle inactivates Mad2, liberating Cdc20 to set off securin degradation.
Later throughout anaphase, APC/C polyubiquitinates mitotic cyclins resulting in their degradation3. Lower in mitotic cyclin-CDK kinase focus results in dephosphorylation of chromosome condensing proteins. The chromosomes decondense and nuclear membranes get re-synthesized. Cells then proceed ahead into telophase the place cytokinesis takes place and the cell cycle completes.

Be taught extra about
References
- Bartek J, Lukas J. (2001). Mammalian G1- and S-phase checkpoints in response to DNA harm. Curr Opin Cell Biol, 13(6), 738-747. doi:10.1016/S0955-0674(00)00280-5
- Vermeulen Okay, Van Bockstaele DR, Berneman ZN. (2003). The cell cycle: a overview of regulation, deregulation and therapeutic targets in most cancers. Cell Prolif, 36(3), 131-149. doi:10.1046/j.1365-2184.2003.00266.x
- Cross FR, Buchler NE, Skotheim JM. (2011). Evolution of networks and sequences in eukaryotic cell cycle management. Philos Trans R Soc Lond B Biol Sci, 366(1584), 3532-3544. doi:10.1098/rstb.2011.0078
- Morgan DO. (1997). Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol, 13, 261-291. doi:10.1146/annurev.cellbio.13.1.261
- Martín Y, Domínguez-Kelly R, Freire R. (2011). Novel insights into sustaining genomic integrity: Wee1 regulating Mus81/Eme1. Cell Div, 6, 2-5. doi:10.1186/1747-1028-6-21
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. (1993). Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement within the self-amplification of MPF at mitosis. EMBO J, 12(1), 53-63. https://pubmed.ncbi.nlm.nih.gov/8428594.
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth Okay. (1998). An ESP1/PDS1 advanced regulates lack of sister chromatid cohesion on the metaphase to anaphase transition in yeast. Cell, 93(6), 1067-1076. doi:10.1016/S0092-8674(00)81211-8
Abbreviations
APC/C: Anaphase selling advanced/cyclosome
ATM: Ataxia telangiectasia mutated kinase
ATP: Adenosine triphosphate
ATR: Ataxia telangiectasia associated kinase
Cdc20: Cell division cycle protein 20
Cdc25: Cell division cycle protein 25
CDKs: Cyclin-dependent kinases
Chk: Checkpoint kinase
DNA: Deoxyribonucleic acid
Mad2: Mitotic arrest poor 2
Mdm2: Murine double minute 2
MPF: Maturation-promoting issue
PLK1: Polo-like kinase 1
Rb: Retinoblastoma
SCF: Skp 1-Cul 1-Fbox protein



