Folding and meeting of multi-protein complicated co-translationally or post-translationally is facilitated by the motion of specialised proteins i.e. molecular chaperons. Throughout the synthesis of polypeptides, among the fraction of polypeptides launched are within the native conformation subsequently, a single chaperon acts on a number of shopper proteins
Molecular chaperones will be outlined because the specialised proteins that work together with improperly folded polypeptides or partially folded, which corrects folding pathways or offering microenvironments for stabilization of folding intermediates and prevention of protein misfolding and aggregation.
Courses of Molecular chaperones:
There are totally different group of molecular chaperones, together with Hsp60s, Hsp40, Hsp90s and sHsps, and Hsp70 proteins. Two main class are described beneath;
1.The primary class, a household of proteins known as Hsp70.
Hsp70/ Warmth shock proteins
These proteins are extra plentiful in cells confused by elevated temperatures such that named as warmth shock protein. The hsp70 equipment acts early within the lifetime of many proteins (usually earlier than the protein leaves the ribosome. They’ve oblique motion of binding and stabilizing the unfolded or partly folded proteins that stopping these proteins from aggregation and degradation. . In addition they block the folding of some proteins that continues to be unfolded till they’ve been translocated throughout membranes. Some chaperones additionally facilitate the quaternary meeting of oligomeric proteins.
The warmth shock proteins bind and launch polypeptides in a cycle involving a number of different proteins (together with a category known as Hsp40) and ATP hydrolysis. They share an affinity for the uncovered hydrophobic patches on incompletely folded proteins. These proteins acknowledge a small stretch of hydrophobic amino acids on a protein’s floor. Aided by a set of smaller hsp40 proteins, ATP-bound hsp70 molecules grasp their goal protein after which hydrolyze ATP to ADP, present process conformational adjustments. This trigger the hsp70 molecules to bind extra tightly with the goal. After the hsp40 dissociates, the speedy rebinding of ATP thus, induces the dissociation of the hsp70 protein after ADP launch. Repeated cycles of hsp binding and launch assist the goal protein to refold.
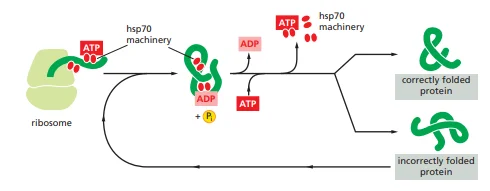

Determine 1: The hsp70 household of molecular chaperones.
[Source; Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Molecular Biology of the Cell. New York: Garland Science]
2.The second class of chaperones is named Chaperonins (Hsp60)
Chaperonins/Hsp60
Chaperonins, which instantly facilitate the folding of proteins. They “protect” proteins which have been denatured by warmth and peptides which are being synthesized (and will not be but folded).In addition they assist to elaborate protein complexes which are required for the folding of numerous mobile proteins which don’t fold appropriately.
About 10% to fifteen% of mobile proteins are recognized to require the chaperonin system, known as GroEL/GroES, for folding below regular situations (as much as 30% require this help when the cells are warmth confused) in E. coli. They had been first recognized to be vital for the expansion of sure bacterial viruses (therefore the designation “Gro”). Unfolded proteins are certain inside pockets within the GroEL complicated, and the pockets are capped transiently by the GroES “lid”. GroES (a co-chaperonin, or hsp10) is a seven-subunit ring that sits on prime of GroEL. GroEL (a chaperonin, or hsp60) consists of two stacked, seven-subunit rings with a cavity wherein ATP-dependent protein folding takes place.
GroEL undergoes s conformational adjustments, coupled to ATP hydrolysis. This causes the binding and launch of GroES, thereby selling folding of the certain polypeptide. Although construction of the GroEL/GroES chaperonin is found, many researches and performance are but to be recognized. Lastly, the folding pathways of numerous proteins require two enzymes that catalyze isomerization reactions.
Protein disulfide isomerase (PDI)
that catalyzes the interchange or shuffling of disulfide bonds for the secure conformation. In addition they catalyze the removing of folding intermediates with inappropriate disulfide cross-links.
Peptideprolyl cis-trans isomerase (PPI)
catalyzes the interconversion of the cis and trans isomers of Professional peptide bonds, which is usually a sluggish step within the folding of proteins that include some Professional residue peptide bonds within the cis conformation.
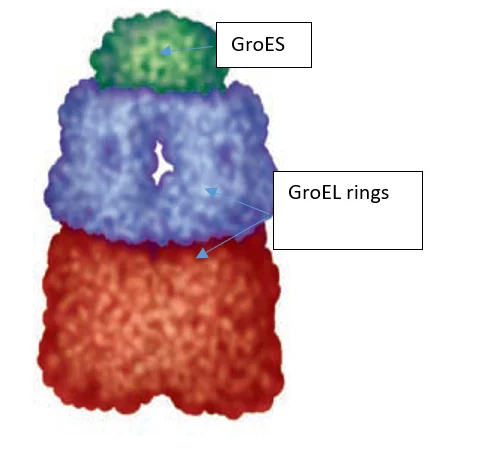

Determine 2:-Area-Filling Mannequin of the E. coli Chaperonin known as the GroESGroEL Complicated
[Source ;Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry].
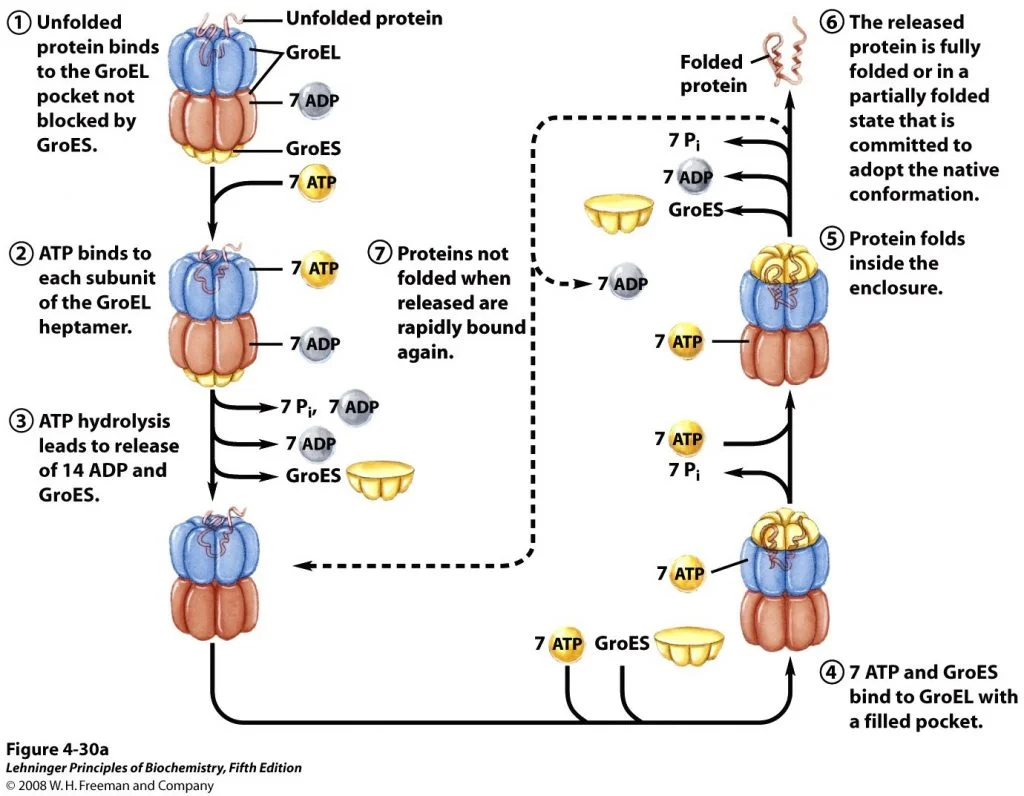

Determine 3; Illustration of Chaperonins Facilitate Folding
[Source ;Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry.]
Roles of chaperons
- “Chaperones,” are required for the right folding of many species of proteins. With out chaperones, a few of these pathways wouldn’t result in the accurately folded in a secure type as a result of the protein would turn into “kinetically trapped” in buildings which are of-pathway. A few of these of-pathway configurations would mixture and be left as irreversible useless ends of nonfunctional and doubtlessly harmful buildings.
- Cells make the most of a number of sorts of chaperones corresponding to hsp as they’re synthesized. As they displays the operation of a suggestions system that responds to a rise in misfolded proteins subsequently it boosts the synthesis of the chaperones that assist these proteins refold.
- Totally different members of those households together with the hsp60 and hsp70 proteins perform in several organelles. Mitochondria include their very own hsp60 and hsp70 molecules which are distinct from those who perform within the cytosol; and a particular hsp70 (known as BIP) helps to fold proteins within the endoplasmic reticulum.
- A few of the chaperones are necessary in preserving the protein unfolded till its synthesis is completed. They act as catalysts by rising the charges of the ultimate phases within the folding course of. Others defend protein fold in order that their susceptible, uncovered areas don’t turn into tangled in unproductive interactions.
References
- Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Ideas Of Biochemistry. New York : W.H. Freeman, 2008 pp151-154
- Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Molecular Biology of the Cell. New York: Garland Science, 2002.pp 354-356
- Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th version. Pp 69-70
- .https://www.nature.com/subjects/chaperones
- https://www.sciencedirect.com/topics/medicine-and-dentistry/chaperone
- https://pdb101.rcsb.org/motm/32
- https://reasonandscience.catsboard.com/t2590p25-origins-what-cause-explains-best-our-existence-and-why
- https://biomedpharmajournal.org/vol4no1/chaperone-co-chaperone-interactions-in-malarial-infection



