The important thing to stopping Alzheimer’s could lie with a girl who by no means had the illness.
Regardless of having a powerful genetic danger, a girl who carried two copies of a uncommon genetic variant linked with late onset Alzheimer’s known as APOE3 Christchurch appeared proof against the illness’s cognitive decline.
Now scientists have noticed how mice with the same set of genetic mutations responded to Alzheimer’s-like circumstances.
Just like the human case, the mice appeared to have fewer neurological defects related to superior levels of the illness, with the important thing issue being how the mind’s cleansing cells (microglia) reply to the illness’s pathology.
This offers recent hope for the event of remedies for Alzheimer’s that target eliciting these explicit responses.
The analysis workforce from the Washington College College of Drugs says this response helps break the connection between the early, symptom-free stage of Alzheimer’s, and the late stage of cognitive decline.
Each genetic – known as autosomal dominant Alzheimer’s illness (ADAD) – and nongenetic types of Alzheimer’s take about 30 years to develop. There are not any signs for the primary 20 years or in order amyloid builds up within the mind slowly.
When amyloid ranges within the mind attain a crucial level, a number of damaging processes start to work collectively. A protein known as tau starts to tangle up and unfold, slowing down the mind’s metabolism and inflicting the tissues to shrink, resulting in cognitive decline.
“One of the biggest unanswered questions in the Alzheimer’s field is why amyloid accumulation leads to tau pathology,” neurologist David Holtzman says.
“Any protective factor is very interesting, because it gives us new clues to how the disease works.”
An prolonged household in Colombia had been coping with ADAD for a lot of generations, with signs starting in half of the members of the family of their 40s. On this household’s case, the illness was triggered by a mutation in a gene known as presenilin-1, related to an elevated tendency to kind amyloid plaques, with amyloid buildup beginning round age 20.
One individual in that household achieved what appeared inconceivable: she stayed cognitively wholesome into her 70s despite the fact that she had inherited the presenilin-1 mutation.
The lady gave the impression to be the one one within the group with two copies of APOE3 Christchurch (APOE3ch) which was proposed as an evidence for her good luck. These carrying solely a single copy of APOE3ch nonetheless exhibited indicators of cognitive deterioration at a youthful age.
A study in 2019 speculated that the girl’s additional mutations have been delaying the method by slowing tau’s fast unfold.
“This woman was very, very unusual in that she had amyloid pathology but not much tau pathology and only very mild cognitive symptoms that came on late,” Holtzman explains. “This suggested to us that she might hold clues to this link between amyloid and tau.”
However since this particular set of genetic mutations has solely been recorded in a single individual on this planet, it has been inconceivable to find out whether or not different components may be concerned in her exceptional cognitive well being.
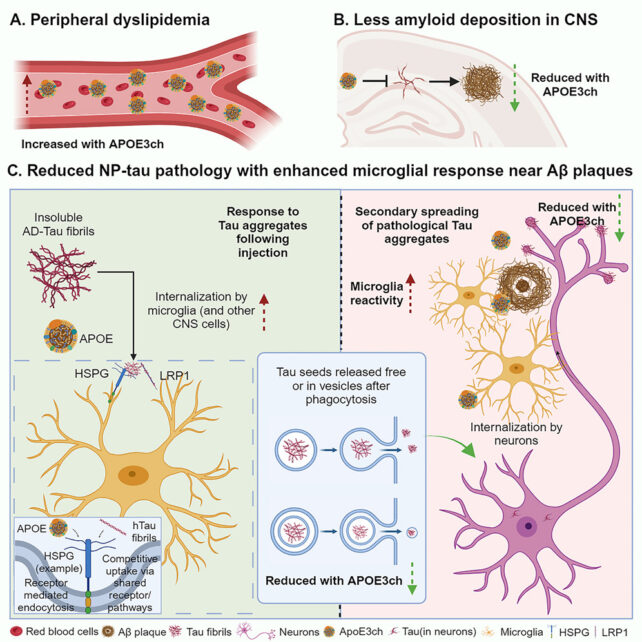
So Holtzman and colleagues studied mice that have been genetically modified to supply extra amyloid, and launched a gene with the APOE3ch mutation. They then injected a small quantity of tau – anticipated to trigger issues in brains already full of amyloid.
Within the mouse fashions, like within the case of the Columbian girl, the tau did not unfold as anticipated. The rationale: microglia across the amyloid plaques have been tremendous energetic and environment friendly in cleansing up the protein.
“These microglia are taking up the tau and degrading it before tau pathology can spread effectively to the next cell,” says Holtzman.
“That blocked much of the downstream process; without tau pathology, you don’t get neurodegeneration, atrophy and cognitive problems.”
The protective effects of APOE3ch aren’t clear in late onset Alzheimer’s though, and could vary depending on a person’s ancestry or if other genetic mutations are involved. This is an important area to be studied further, the team adds.
“If we can find a way to mimic the effects of the APOE Christchurch mutation,” Holtzman says, “we may be able to stop people who already are on the path to Alzheimer’s dementia from continuing down that path.”
The research has been printed in Cell.



